Expert Tips
Calculating the Carbon Stored in Wood Products
There are many complex elements associated with carbon accounting and life cycle assessment (LCA). Fortunately, the amount of carbon stored in wood is a straightforward calculation that remains consistent for each wood species.

As a tree grows, it absorbs carbon dioxide from the atmosphere, stores the carbon in its wood fibers, and releases the oxygen back to the air through the process of photosynthesis.
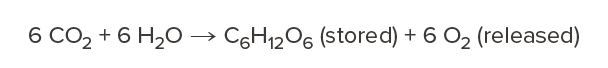
Carbon storage is a unique attribute of wood that does not occur in other structural materials. It’s a natural process available to us now—without waiting for new carbon capture and storage technologies to be developed—allowing us to meet near-term carbon reduction goals and transforming our built environment into carbon sinks instead of sources.1
How much carbon is stored in my wood product?
Although it varies slightly by species, wood is roughly 50% carbon by dry weight. Thus, if you know the density of the wood in question, you can easily calculate its stored carbon.

For example:
At 15% moisture content (MC), Douglas fir-larch weighs 34.5 lb/ft3. To determine the dry weight, divide by 1.15:
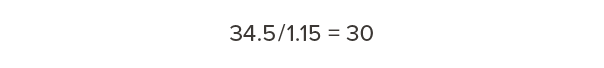
Dry Douglas fir-larch weighs 30 lb/ft3
Half of that dry weight is carbon:
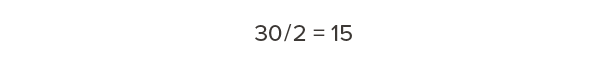
Every cubic foot of Douglas fir-larch contains 15 lb of carbon.
This is true for all Douglas fir-larch, regardless of where or how it is grown. A similar calculation can be made for other species, depending on their density.
How much carbon dioxide is stored in my wood product?
Carbon dioxide isn’t stored in wood, per se. Carbon is stored in wood—and if that carbon were released into the atmosphere, it would combine with oxygen to form carbon dioxide. Therefore, we can calculate and report an equivalent amount of carbon dioxide stored in the wood.
Carbon has a molecular weight of 12. Oxygen has a molecular weight of 16. Therefore, carbon dioxide—one carbon and two oxygens—has a molecular weight of 44.
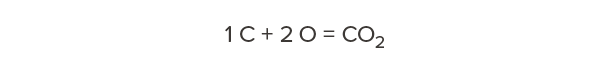
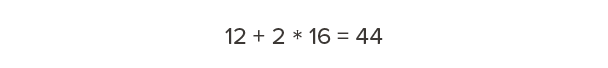
So, for every 12 pounds of carbon we’re storing in wood, that’s equivalent to 44 pounds of carbon dioxide that would otherwise occur in the atmosphere. Our calculations above showed that one cubic foot of Douglas fir-larch contains 15 lb of carbon. How much carbon dioxide is this equivalent to?
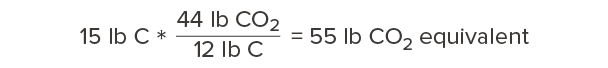
Every cubic foot of Douglas fir-larch stores the equivalent of 55 lb of carbon dioxide!
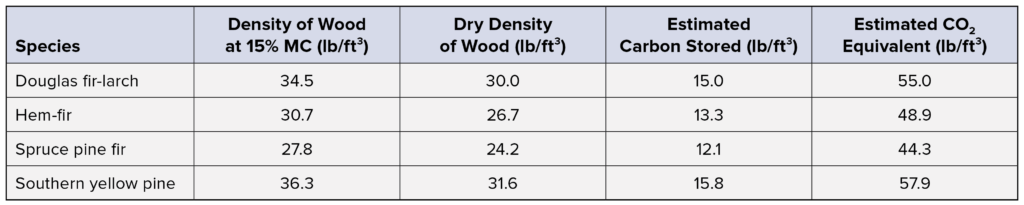
A summary of this data for common structural wood species is provided in the table below. For reference, burning one gallon of gasoline produces about 20 lb of carbon dioxide.2
Adapted from:
Dovetail Partners, Inc. (2013). Carbon in Wood Products – The Basics.
1 Think Wood. Carbon Source to Carbon Sink: Redesigning the Built Environment for Climate Change.


